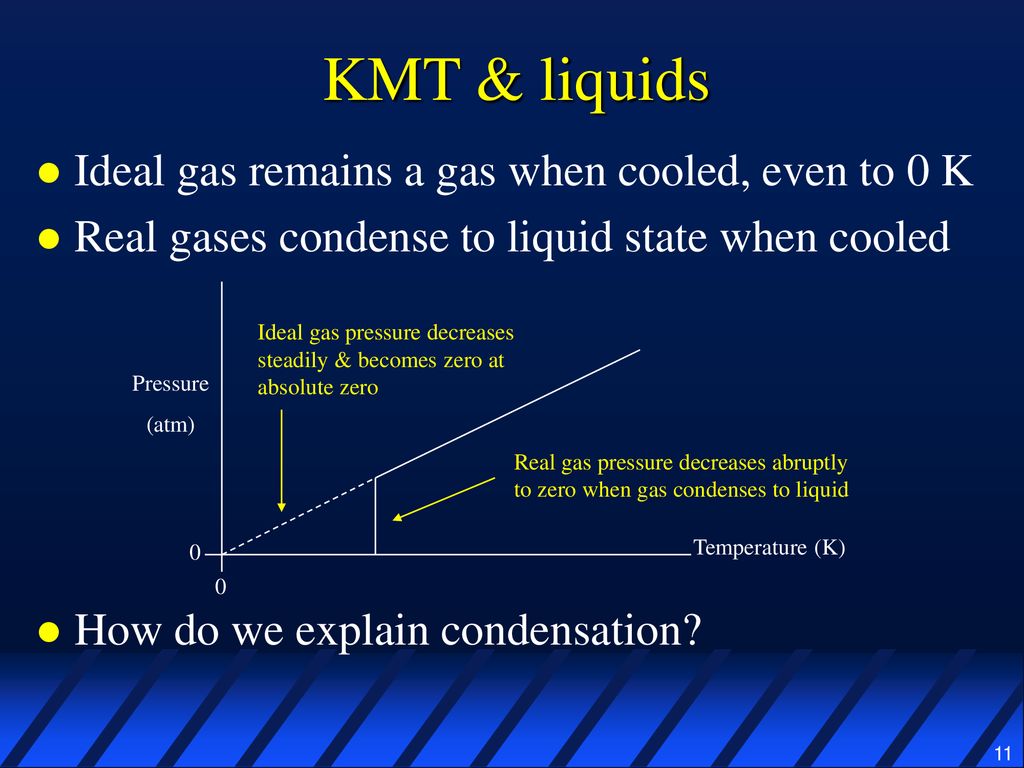
Do Gases Condense To Form Liquids When Cooled . For example, liquid water turns into steam when it is heated. some substances turn directly from gas to solid when cooled, and this too is called subliming. these changes are reversible, too. Because they no longer have enough energy to escape. When the gas is cooled, the vapour condenses to make it a liquid again. water vapour molecules in the air collide with the cool surface of the glass and lose their kinetic energy. substances can change state, usually when they are heated or cooled. learn how real gases undergo a phase transition from gas to liquid as the temperature is lowered and the pressure. Cool that liquid down even. if you lower the temperature enough and remove enough energy from the particles, every gas will sooner or later condense to a liquid. learn how cooling water vapor causes condensation, the process in which molecules of a gas form a liquid.
from slideplayer.com
if you lower the temperature enough and remove enough energy from the particles, every gas will sooner or later condense to a liquid. When the gas is cooled, the vapour condenses to make it a liquid again. these changes are reversible, too. some substances turn directly from gas to solid when cooled, and this too is called subliming. Because they no longer have enough energy to escape. learn how cooling water vapor causes condensation, the process in which molecules of a gas form a liquid. For example, liquid water turns into steam when it is heated. water vapour molecules in the air collide with the cool surface of the glass and lose their kinetic energy. substances can change state, usually when they are heated or cooled. Cool that liquid down even.
Liquids & Aqueous solutions ppt download
Do Gases Condense To Form Liquids When Cooled Cool that liquid down even. For example, liquid water turns into steam when it is heated. water vapour molecules in the air collide with the cool surface of the glass and lose their kinetic energy. When the gas is cooled, the vapour condenses to make it a liquid again. if you lower the temperature enough and remove enough energy from the particles, every gas will sooner or later condense to a liquid. some substances turn directly from gas to solid when cooled, and this too is called subliming. substances can change state, usually when they are heated or cooled. learn how real gases undergo a phase transition from gas to liquid as the temperature is lowered and the pressure. Because they no longer have enough energy to escape. learn how cooling water vapor causes condensation, the process in which molecules of a gas form a liquid. Cool that liquid down even. these changes are reversible, too.
From www.snexplores.org
Explainer What are the different states of matter? Do Gases Condense To Form Liquids When Cooled some substances turn directly from gas to solid when cooled, and this too is called subliming. water vapour molecules in the air collide with the cool surface of the glass and lose their kinetic energy. Cool that liquid down even. learn how real gases undergo a phase transition from gas to liquid as the temperature is lowered. Do Gases Condense To Form Liquids When Cooled.
From slideplayer.com
Big Idea 9 Changes in Matter ppt download Do Gases Condense To Form Liquids When Cooled For example, liquid water turns into steam when it is heated. these changes are reversible, too. if you lower the temperature enough and remove enough energy from the particles, every gas will sooner or later condense to a liquid. substances can change state, usually when they are heated or cooled. learn how cooling water vapor causes. Do Gases Condense To Form Liquids When Cooled.
From kids.britannica.com
evaporation and condensation Kids Britannica Kids Homework Help Do Gases Condense To Form Liquids When Cooled For example, liquid water turns into steam when it is heated. Cool that liquid down even. substances can change state, usually when they are heated or cooled. learn how cooling water vapor causes condensation, the process in which molecules of a gas form a liquid. Because they no longer have enough energy to escape. if you lower. Do Gases Condense To Form Liquids When Cooled.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning Do Gases Condense To Form Liquids When Cooled substances can change state, usually when they are heated or cooled. some substances turn directly from gas to solid when cooled, and this too is called subliming. if you lower the temperature enough and remove enough energy from the particles, every gas will sooner or later condense to a liquid. water vapour molecules in the air. Do Gases Condense To Form Liquids When Cooled.
From circuitdbseriatim.z13.web.core.windows.net
Solid Liquid Gas Diagram Do Gases Condense To Form Liquids When Cooled learn how real gases undergo a phase transition from gas to liquid as the temperature is lowered and the pressure. learn how cooling water vapor causes condensation, the process in which molecules of a gas form a liquid. if you lower the temperature enough and remove enough energy from the particles, every gas will sooner or later. Do Gases Condense To Form Liquids When Cooled.
From slideplayer.com
MATTER AND ATOMIC STRUCTURE matter = anything that takes up space and Do Gases Condense To Form Liquids When Cooled some substances turn directly from gas to solid when cooled, and this too is called subliming. substances can change state, usually when they are heated or cooled. Because they no longer have enough energy to escape. water vapour molecules in the air collide with the cool surface of the glass and lose their kinetic energy. Cool that. Do Gases Condense To Form Liquids When Cooled.
From slideplayer.com
Unit 1 Lesson 6 Changes of State ppt download Do Gases Condense To Form Liquids When Cooled learn how cooling water vapor causes condensation, the process in which molecules of a gas form a liquid. substances can change state, usually when they are heated or cooled. some substances turn directly from gas to solid when cooled, and this too is called subliming. water vapour molecules in the air collide with the cool surface. Do Gases Condense To Form Liquids When Cooled.
From www.slideserve.com
PPT Chapter 23 Changes of Phase PowerPoint Presentation ID779795 Do Gases Condense To Form Liquids When Cooled these changes are reversible, too. learn how cooling water vapor causes condensation, the process in which molecules of a gas form a liquid. Cool that liquid down even. learn how real gases undergo a phase transition from gas to liquid as the temperature is lowered and the pressure. Because they no longer have enough energy to escape.. Do Gases Condense To Form Liquids When Cooled.
From circuitlibrarybauer.z13.web.core.windows.net
Solid Liquid Gas Diagram Do Gases Condense To Form Liquids When Cooled When the gas is cooled, the vapour condenses to make it a liquid again. these changes are reversible, too. if you lower the temperature enough and remove enough energy from the particles, every gas will sooner or later condense to a liquid. Cool that liquid down even. For example, liquid water turns into steam when it is heated.. Do Gases Condense To Form Liquids When Cooled.
From circuitgonelladrianxm.z22.web.core.windows.net
Phase Diagram Phase Changes Do Gases Condense To Form Liquids When Cooled water vapour molecules in the air collide with the cool surface of the glass and lose their kinetic energy. Cool that liquid down even. learn how cooling water vapor causes condensation, the process in which molecules of a gas form a liquid. substances can change state, usually when they are heated or cooled. these changes are. Do Gases Condense To Form Liquids When Cooled.
From slideplayer.com
By Tyler Hamilton Erin Wolff ppt download Do Gases Condense To Form Liquids When Cooled Cool that liquid down even. When the gas is cooled, the vapour condenses to make it a liquid again. Because they no longer have enough energy to escape. some substances turn directly from gas to solid when cooled, and this too is called subliming. these changes are reversible, too. For example, liquid water turns into steam when it. Do Gases Condense To Form Liquids When Cooled.
From exohzkqeb.blob.core.windows.net
Do Gases Condense To Form Liquids at Yolanda Cortez blog Do Gases Condense To Form Liquids When Cooled these changes are reversible, too. some substances turn directly from gas to solid when cooled, and this too is called subliming. water vapour molecules in the air collide with the cool surface of the glass and lose their kinetic energy. learn how cooling water vapor causes condensation, the process in which molecules of a gas form. Do Gases Condense To Form Liquids When Cooled.
From stock.adobe.com
Vector scientific illustration of changing states of matter from liquid Do Gases Condense To Form Liquids When Cooled water vapour molecules in the air collide with the cool surface of the glass and lose their kinetic energy. substances can change state, usually when they are heated or cooled. learn how real gases undergo a phase transition from gas to liquid as the temperature is lowered and the pressure. learn how cooling water vapor causes. Do Gases Condense To Form Liquids When Cooled.
From apollo.lsc.vsc.edu
Latent Heats sublimation and deposition Do Gases Condense To Form Liquids When Cooled Cool that liquid down even. learn how cooling water vapor causes condensation, the process in which molecules of a gas form a liquid. some substances turn directly from gas to solid when cooled, and this too is called subliming. if you lower the temperature enough and remove enough energy from the particles, every gas will sooner or. Do Gases Condense To Form Liquids When Cooled.
From www.numerade.com
SOLVED Why gases condense when they are cooled? Do Gases Condense To Form Liquids When Cooled When the gas is cooled, the vapour condenses to make it a liquid again. For example, liquid water turns into steam when it is heated. learn how cooling water vapor causes condensation, the process in which molecules of a gas form a liquid. these changes are reversible, too. some substances turn directly from gas to solid when. Do Gases Condense To Form Liquids When Cooled.
From exohzkqeb.blob.core.windows.net
Do Gases Condense To Form Liquids at Yolanda Cortez blog Do Gases Condense To Form Liquids When Cooled learn how real gases undergo a phase transition from gas to liquid as the temperature is lowered and the pressure. learn how cooling water vapor causes condensation, the process in which molecules of a gas form a liquid. Cool that liquid down even. When the gas is cooled, the vapour condenses to make it a liquid again. . Do Gases Condense To Form Liquids When Cooled.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Do Gases Condense To Form Liquids When Cooled learn how cooling water vapor causes condensation, the process in which molecules of a gas form a liquid. these changes are reversible, too. water vapour molecules in the air collide with the cool surface of the glass and lose their kinetic energy. Cool that liquid down even. learn how real gases undergo a phase transition from. Do Gases Condense To Form Liquids When Cooled.
From www.slideserve.com
PPT Cool Condensation PowerPoint Presentation, free download ID2835918 Do Gases Condense To Form Liquids When Cooled For example, liquid water turns into steam when it is heated. if you lower the temperature enough and remove enough energy from the particles, every gas will sooner or later condense to a liquid. some substances turn directly from gas to solid when cooled, and this too is called subliming. substances can change state, usually when they. Do Gases Condense To Form Liquids When Cooled.